Shark Ecology in the Mid-Atlantic Bight
Assessing habitat-use, foraging dynamics, ecotoxicology, stress physiology, and health of migratory sharks in the continental shelf waters of New York and New Jersey
Project Investigators
- Oliver N. Shipley1
- Michael G. Frisk1
- Jill A. Olin1,2
- Gregory Henkes3
- Merry Camhi4
- Keith. J. Dunton5
Collaborating Partners
- Lisa Crawford1
- Alisa (Harley) Newton6
- Michael Hyatt6
- Hans Walters6
- Jake LaBelle6
- Ryan Knotek7
(1) School of Marine & Atmospheric Sciences, Stony Brook University, Stony Brook, 11794 NY, USA.
(2) Great Lakes Research Center, Michigan Technology University, Houghton, 49931 MI, USA.
(3) Department of Geosciences for Earth & Planetary Science, Stony Brook University, Stony Brook, 11794 NY, USA.
(4) Wildlife Conservation Society, Bronx, 10460 NY, USA
(5) Department of Biology, Monmouth University, West Long Branch, 07764 NJ, USA.
(6) New York Seascape Program, The New York Aquarium, Bronx, 10460, NY, USA.
(7) University of University of Massachusetts Boston, Boston, 02125, MA, USA.
Background
Over the last sixty years, global shark populations have exhibited unprecedented declines, owing largely to targeted and incidental fisheries exploitation (Ferretti et al. 2010; Dulvy et al. 2014; Roff et al. 2018). Such declines are of increasing conservation concern to managers and policy makers, as sharks serve as an important structural component of global marine ecosystems (Camhi et al. 2008, 2009; Hammerschlag et al. 2019). Highly migratory sharks represent a particularly vulnerable demographic, as extensive and often complex movement patterns increase their susceptibility to fisheries interactions, often across multiple jurisdictions (Mandelman et al. 2008; Graham et al. 2016; Queiroz et al. 2019). For many highly migratory sharks, management approaches can be challenged by knowledge gaps regarding aspects of their biology; for example, common migration corridors, the location of important foraging grounds, and the behavioral and physiological response of individuals to fisheries capture (both commercial and recreational) are poorly defined for many species. Improving our knowledge of these facets will allow managers to more effectively assess the potential species overlap with areas of high fishing (Queiroz et al. 2016) and mitigate fisheries interactions, as well as help protect important resource pools that support diverse shark assemblages. These data can inform population assessments and facilitate management to ensure long-term sustainability of local shark populations.
Throughout the summer months, the waters of the Mid-Atlantic Bight (MAB), specifically New York and New Jersey support vulnerable shark species during their extensive migratory circuits (Vaudo et al. 2017; Howey et al. 2017), many of which have received increasing conservation concern over recent years (Cailliet et al. 2009; Musick et al. 2009; Stevens 2009; Goldman et al. 2009). Primarily, Sandbar Sharks (Carcharhinus plumbeus), Sand Tiger Sharks (Carcharias taurus), Shortfin Mako Sharks (Isurus oxyrinchus), Dusky Sharks (Carcharhinus obscurus), Blue Sharks (Prionace glauca), Blacktip Sharks (Carcharhinus limbatus), White Sharks (Carcharodon carcharias), and Common Thresher Sharks (Alopias vulpinus) comprise the pelagic shark assemblage in this region. Despite their seasonal residence and long history of supporting recreational fisheries, biological information pertaining to these species is severely lacking in this region. Therefore, providing new information on migration, foraging, health, and capture behavior and physiology will improve our understanding of the mechanisms supporting the seasonal presence of these vulnerable species, and allow for a more comprehensive management to be established in this region.
Research Goals
Through an extensive collaborative network, the overarching goal of this study is to improve knowledge of poorly studied, but threatened, migratory sharks in the MAB (New York and New Jersey). Through application of passive and near real-time tracking technologies and a suite of biochemical assays, we aim to define fine-scale movement patterns, identify common geographical foraging hotspots, examine contaminant burdens, assess physiological responses of animals to capture events, and provide a general health assessment. Target species include, but are not limited to the Blue Sharks, Shortfin Mako Sharks, Blacktip Sharks, Dusky Sharks, Sandbar Sharks, Sand Tiger Sharks, and Common Thresher Sharks.
Research Objectives
- Examine the timing and extent of seasonal movements to identify potential migration corridors for each species.
- Identify the ecological role of sharks and identify potentially important foraging grounds supporting species biomass.
- Measure concentrations of potentially harmful contaminants present in non-destructively sampled tissues.
- Investigate relationships between contaminant bioaccumulation and altered cellular pathways.
Provide overall health assessments for sharks. - Assess the physiological and behavioral response of species to recreational fisheries capture.
Research Results General Summary
The science team implemented their research aboard the M/V Albula during three cruises in the summer of 2019 in waters of the MAB off New Jersey (July 23-July 29) and New York (August 1-8; 9-16). Fishing occurred a total of 18 days within the cruise window, whereby the team created a chum slick and used static rod and reel angling to catch sharks. Fishing locations were based on local fishers’ knowledge. In New Jersey, fishing was focused around the ‘Mud Hole’ a region that marks the entrance to the Hudson Canyon, known to support lucrative fishing grounds throughout the spring and summer months (Fig. 1). This area is characterized by deep channels and shallow drop offs, providing structure for both forage fish and higher predators. In New York, fishing was conducted approximately 15 nm offshore of the SS Oregon shipwreck. This region has proven productive for the science team in the past two fishing seasons, and the location was provided by recreational anglers that have been fishing NY waters for decades (Fig. 1). Initially, the science team proposed to sample three areas within the MAB, including offshore of New Jersey, and offshore of Fire Island and Montauk, NY. However, given the success with sampling during the first two cruises and weather considerations, the science team did not pursue fishing efforts off Montauk, NY.
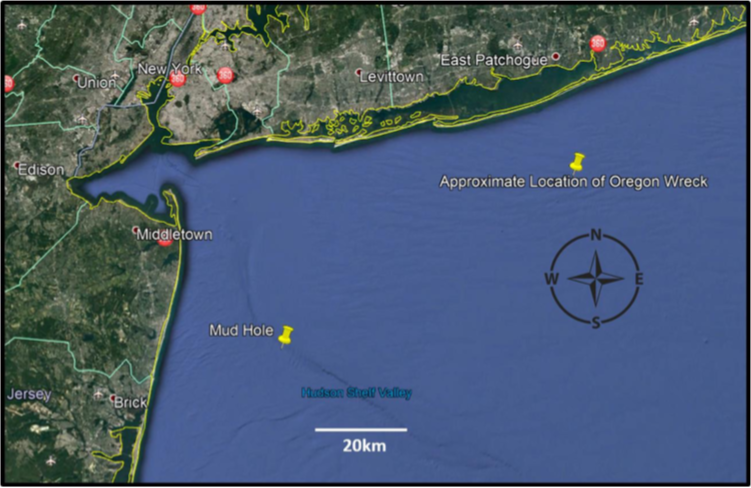
Figure 1. Offshore areas in the MAB targeting for sampling the pelagic shark assemblage during summer 2019 aboard the M/V Albula.
The research team successfully captured and collected biological information on 32 individuals spanning 6 species including: Blacktip Sharks, Dusky Sharks, Sandbar Sharks, Sand Tiger Sharks, Shortfin Mako Sharks, and Blue Sharks. Once hooks were set, sharks were brought to the side of the boat, secured and immediately placed into tonic immobility (i.e., state of ‘sleep’ by turning individuals upside down), to allow for the internal transplanting of an acoustic transmitter, as well as for a blood and fecal samples to be taken. Animals were then turned upright, and seven morphometric measurements were taken in order to derive body condition. Dorsal white muscle and fin clips were taken from each animal, before an external, unique identification tag was inserted into the dorsal musculature. For Shortfin Mako Sharks only, a fin-mount satellite tag was affixed to the dorsal fin (n = 1). Prior to release, animals were bled for a second time, before the entire hook was removed and the animal released. Generally, this precedure did not exceed 15 minutes. Tissue samples for foraging dynamics and stress physiology were stored in liquid nitrogen upon sampling and will be processed for question-
specific data in the coming months. Samples for health assessments were processed immediately onboard the M/V Albula. Summary information from sampled animals is provided in Table 1 and discussed below.
Movement and Habitat-use
In the western North Atlantic Ocean, Shortfin Mako Sharks range from northeast of the Grand Banks south to Florida, the Caribbean and the Gulf of Mexico (Casey & Kohler 1992). Vaudo et al. (2017) identified the Mid-Atlantic Bight as a core region of distribution for this species. Shortfin Mako Sharks spend a significant amount of time at the ocean’s surface (Vaudo et al. 2016) so are ideal candidates for near-real time satellite transmitters. Their long-range seasonal movements may be influenced by ambient water temperature (Casey & Kohler 1992), but prey movement/availability may also affect movement in this endothermic lamnid (Vaudo et al. 2017). On 4 August 2019, the science team tagged a 137 cm (FL) juvenile male Shortfin Mako with a single-point Smart Position Only Transmitting (SPOT) tag from Wildlife Computers, Inc (Redmond, WA). The team attached the tag to the distal portion of the first dorsal fin via two 10- 24 threaded nylon rods inserted into holes drilled in the fin; the tag was secured to the fin with type 316 stainless steel 10-24 lock nuts threaded onto the rods. Since its release, this individual has provided daily geolocation estimates, with location quality occasionally hampered by rough seas or other weather events (e.g., high cloud coverage). After tagging, the individual moved toward the Hudson Canyon before moving to the New York Bight continental shelf edge boundary (Fig. 2).
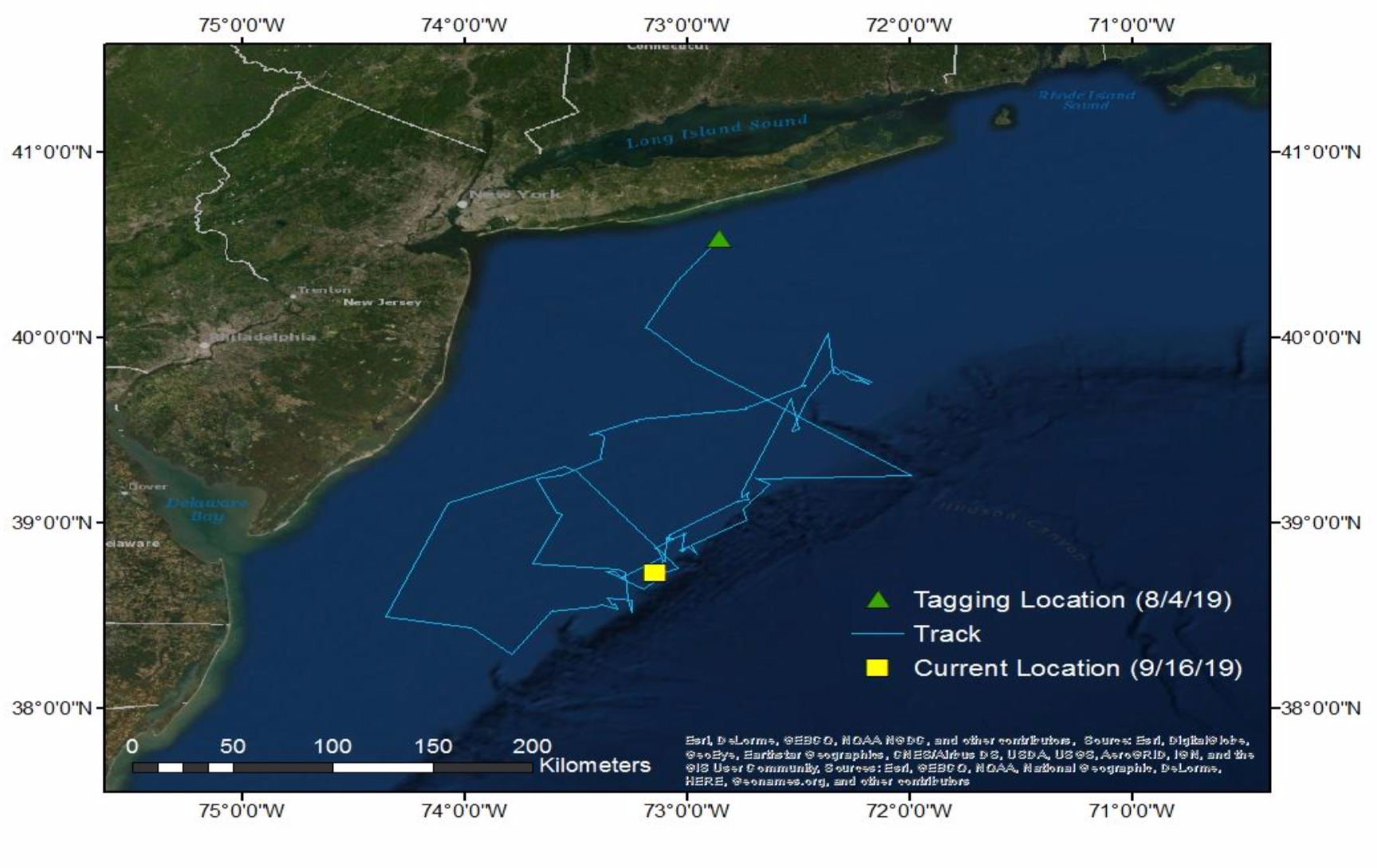
Figure 2. Movement patterns of the juvenile male Shortfin Mako tagged off Fire Island, NY on 4 August 2019.
In addition to the deployment of satellite tags, internal acoustic transmitters (Vemco V16-6H; 69 kHz; high-power output=158 dB re 1lPa at1 m; random transmitter delay= 70–150 s [2,331-d tag life]) were deployed by surgically implanting the tags, which will provide fine-scale movement information for the next 7-10 years. We tagged a total of 25 sharks from five species with internal acoustic transmitters (Table 1). PI Frisk currently maintains an extensive array of listening stations throughout coastal New York and New Jersey, to track the fine-scale movements of individuals throughout this area (Fig 3). Additionally, PI Frisk participates in the Atlantic Cooperative Telemetry (ACT) Network (www.theactnetwork.com), a network of researchers from several institutions/agencies that maintain arrays of Vemco acoustic receivers in numerous locations along the coast, whereby members of the network agree to share detection information of tagged individuals.
Data from PI Frisk’s acoustic array (Fig. 3) has been used to document movements of White Sharks (Carcharodon carcharias) (Curtis et al. 2019), Atlantic Sturgeon (Melnychuk et al. 2017; Ingram et al., 2019), and Winter Skate ( Frisk et al. 2019). Using these data, Frisk et al. showed weekly mortality estimates in Atlantic Sturgeon and identified the locations and times when bycatch events occurred (Melnychuk et al. 2017). Moreover, when combined with partner array networks long distance movements in Atlantic Sturgeon (FL to Canada, K. Dunton, unpublished data) and Winter Skate (NC to MA, Frisk et al. 2019) have been observed. We expect that over the coming years we will be able to define timing and extent of migration occurring for our focal species.
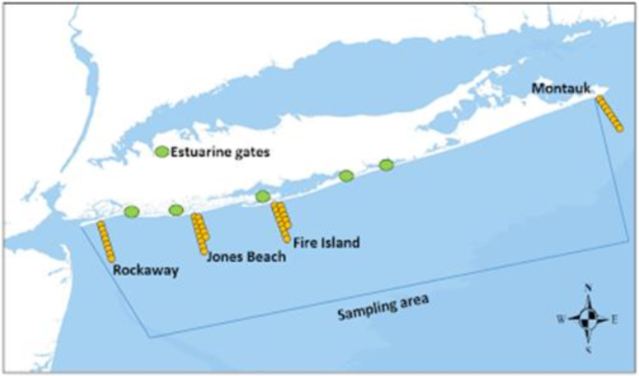
Figure 3. Location of the acoustic array maintained by PI Frisk. This array has been deployed for 7 years (orange dots). Estuarine gates (green circles) were installed in 2019. Funding for these arrays is secured until 2024.
Foraging Dynamics
The foraging dynamics of sharks directly structure marine food-web dynamics, through processes such as top-down control of prey populations (Hammerschlag et al. 2019) and facilitating ecosystem connectivity (McCauley et al. 2012; Shipley et al. 2019). A common method to quantify these interactions in wild populations, is through analysis of naturally occurring stable isotope ratios of carbon (δ13C) and nitrogen (δ15N), which can be used to trace primary production sources that underpin shark biomass, as well infer trophic diversity exhibited by individuals in a food-web (Hussey et al. 2012; Shipley et al. 2017). Combined δ13C and δ15N can be used to infer competitive interactions (or lack thereof) for resources within co-occurring shark populations. Sampling tissues of varying metabolic activity also provides a temporal resolution that cannot be achieved using traditional dietary methods, such as stomach content analysis. Generally, muscle tissue provides a time-integrated average of trophic behavior occurring across ~1 year (Kim et al. 2012), whereas blood plasma provides more recent information spanning approximately 60 days (Zeichner et al. 2017). This information is vital to establishing the functional role of highly migratory sharks within New York food-webs, and as a result predict species and ecosystem-level responses to continued population declines. Tissue samples (see Table 1), including muscle and plasma, will be processed for bulk stable isotopes of carbon and nitrogen in the coming months. These data will be integrated with existing data analyzed from sampling seasons from 2017 and 2018 to provide a temporal understanding of foraging dynamics of these species in the MAB. Stable isotope data derived from 2017 and 2018 sampling efforts illustrate the range in trophic positions (Fig. 4 bottom panel) among our focal species, with Common Thresher Sharks at the top of the food web relative to Sandbar and Shortfin Mako Sharks. Stable isotopes of blood plasma (weeks) indicate that Sandbar Sharks do not appear to share resources with Common Thresher Sharks during periods when they frequent NY and NJ waters, but stable isotopes of red blood cells (months) do show an overlap in resource use of these species during periods outside of NY/NJ waters (Fig. 4 top panel). Data collected aboard the M/V Albula during summer 2019 increases sample sizes for several focal species, but also provides data on new species (e.g., Blue Sharks, Blacktip Sharks, and Sand Tiger Sharks) within the assemblage that have yet to be analyzed for this region.
Current stable isotope data (2017-2018) of our focal species suggests that co-occurrence may be facilitated by a diverse and productive prey base. However, to date, the prey base has not been sampled to confirm. Moving forward, however, tissues (i.e., blood, muscle and feces) sampled from individuals in 2019 will be further analyzed using fatty acid analysis and DNA metabarcoding techniques to identify specific prey resources used during the period within the study area, building upon preliminary findings. Sampling of tissues toward this objective was advantageous during this research cruise and will allow for my fine-scale evaluation of temporal predator-prey dynamics. These data will be coupled with stable isotope data, which will be processed and analyzed in the coming months at Stony Brook University. This project component will ultimately provide some of the first information on resource use dynamics and energy pathways for shark species residing in the MAB. This will allow for the ecological role of these species to be established for models of MAB food-webs, and thus directly inform ecosystem-based management for this region.
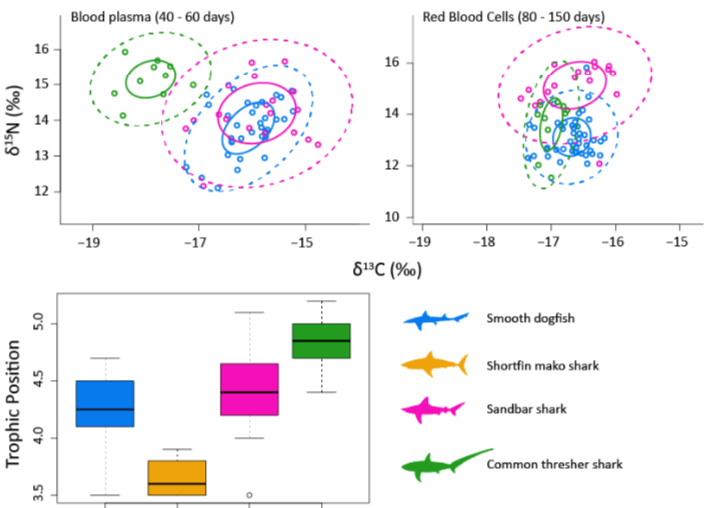
Figure 4. Top panels: isotopic niches for Smooth Dogfish, Sandbar Sharks, and Common Thresher sharks sampled from coastal New York. Isotope data is generated from two tissue types of varying isotopic turnover rate (blood plasma and red blood cells), to examine changes in niche dynamics over time. Bottom panel: trophic position estimates derived from δ15N values of plasma tissue sampled during periods when animals are frequenting coastal New York.
Ecotoxicology
Sharks are slow-growing, long-lived, slow to sexually mature, have long gestation periods, produce few offspring, and have large fatty livers (Maz-Courrau et al. 2012). These life history characteristics make them vulnerable to environmental stressors including chemical pollutants that bioaccumulate such as mercury (THg). Effects of high THg in teleost fish include reproductive impairment (as reviewed by Crump & Trudeau 2009), behavioral alterations, and diminished growth (as reviewed by Gelsleichter & Walker 2010), but such effects are poorly known for sharks. To date, THg concentrations have been reported in a number of shark species (e.g., Adams & McMichael 1999; Mull et al. 2012; Le Bourg et al. 2014; Kiszka et al. 2015; Matulik et al. 2017; O’Bryhim et al. 2017), but assessments of the associated physiological and health effects have yet to be evaluated. This is particularly pertinent for our focal species, including Shortfin Mako Sharks and Common Thresher Sharks that are commonly consumed by humans throughout the MAB. Muscle tissue samples (Table 1) will be analyzed for THg and for physiological responses to THg exposure using RNA as a proxy. Specifically, THg concentrations will be obtained using a direct mercury analyzer (DMA-80) and RNA will be extracted from muscle tissue and quantitative PCR assay will be used to identify differential RNA expression of a suite of transcripts known to be associated with organic contaminant exposure and effects. These data will be integrated with existing data analyzed from sampling seasons from 2017 and 2018.
THg concentrations of sharks sampled in 2017 and 2018 show that Sandbar and Shortfin Mako sharks possessed similar THg concentrations, with both having higher concentrations than Sand Tiger Sharks (Fig. 5 left panel). THg concentrations appear to increase with length in both Shortfin Mako and Sand Tiger Shark, suggesting that individuals bioaccumulate THg as they grow larger (Fig. 5 right panel). Relationships for sandbar sharks are harder to interpret, but preliminary data suggests that bioaccumulation of THg is not linear, and obvious trends may be impacted by physiological processes such as growth dilution. Data across a greater size range, however, is required to support this hypothesis. Samples collected aboard the M/V Albula will not only help refine hypotheses relating to trophic transfer of THg throughout marine food-webs of the MAB, and the associated effects that this may have for shark health, but provide a broader size-range of individuals to evaluate mechanisms of THg bioaccumulation, such as habitat and ontogenetic shifts in life-history (Merson & Pratt 2001; Rechisky & Wetherbee et al. 2003; Engle et al. 2010; Kwon et al. 2014; Lyons et al. 2019). In addition, continued efforts also have consequential implications for human health as measured concentrations are above guidelines recommended for human consumption by the Environmental Protection Agency (EPA, 0.3 ppm, ww), and Food and Drug Administration (FDA, 1.0 ppm, ww).
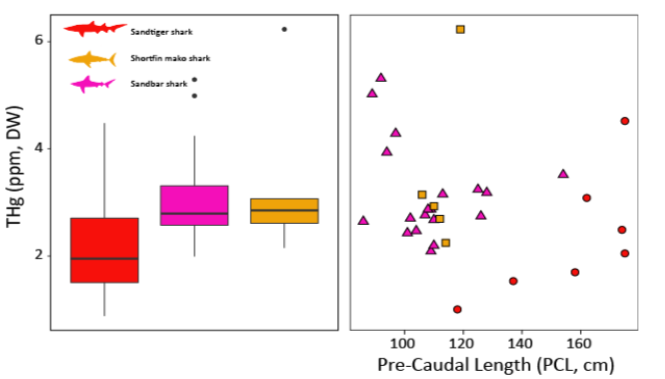
Figure 5. Left panel: Comparison of total mercury (THg) concentration in muscle tissue in three shark species, Sand Tiger (n = 7), Sandbar (n = 19), and Shortfin Mako (n = 5) Sharks. Right panel: Relationships between precaudal length and THg concentration in muscle tissue, demonstrating that sharks bioaccumulate mercury as they grow.
Health Assessments
Health assessments including external physical evaluation and blood work were performed on 28 animals (see Table 1). Baseline blood work collected consisted of packed cell volume (PCV), total solids (TS), hemoglobin levels (HgB), buffy coat percentage (BC), white blood cell (WBC) count, red blood cell (RBC) count, blood smear review, WBC differential count, plasma chemistry, plasma protein electrophoresis, and acute phase proteins. Blood work completed to date includes PCV, TS, HgB, BC, blood smear review and complete blood counts (WBC, RBC) for all species. Differential counts, plasma chemistry, protein electrophoresis and acute phase protein testing are pending completion.
Nine animals (32%) had clinical abnormalities noted during physical examination (Table 2). This included external skin lesions that were likely due to infectious/inflammatory causes (n = 3), abrasions and bite wounds due to nonspecific or possibly conspecific trauma (n = 3), trauma due to fisheries interactions (gill net entanglement, residual J hook and monofilament) (n = 2) and trauma due to anthropogenic agents (plastic entanglement) (n = 1). An additional five animals (18%) were found to have hematologic abnormalities without any external clinical abnormalities. This includes significant anemia in one sandbar shark (PCV 10.5%; RBC count: 203K cells/ul) and four sandbar sharks with hemoparasitism. Among animals with abnormalities on physical examination, significant corresponding hematologic changes were only found in the two sandbar sharks with lacerations due to gill net and plastic entanglement respectively. Both animals had significantly elevated white blood cell counts compared to published normal ranges and other animals examined on this trip with normal physical exams. All other animals had normal hematologic values.
The Sandbar Shark hemoparasite detected is an intranuclear organism and appears to be primarily within monocytes and large white blood cell precursors (Table 3, Fig. 6). It was detected in three animals sampled during the New Jersey leg of the trip, and one animal during the New York leg of the trip. This is a novel parasite that we have detected on only one occasion previously in a Sandbar Shark in the New York Bight in 2017. Electron microscopy previously confirmed this organism to be an intranuclear microsporidian and results from PCR and next generation sequencing identified the organism as a new species, Nucleospora sp. nov. Nucleospora causes a leukemia like illness in teleost species, but to our knowledge it has never been reported in elasmobranchs. Interestingly WBC counts in the affected animals were within published ranges of normal. Differential counts, which discern the distribution of WBC types in the sample, may indicate abnormalities when completed. Samples collected from the current expedition will be invaluable in further documenting this organism in the species and will assist us in determining the potential clinical impact on Sandbar Sharks.
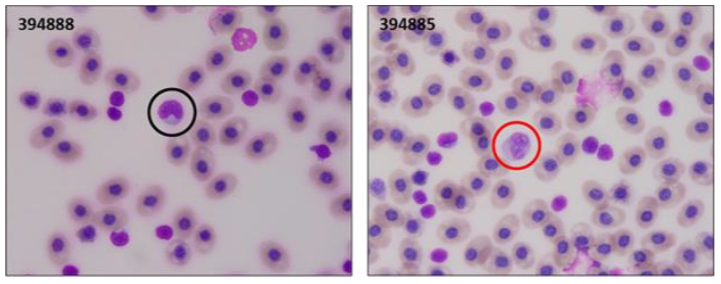
Figure 6. Left panel: Normal Sandbar Shark (394888) monocyte (black circle); Right panel: Sandbar Shark (394885) monocyte with intranuclear microsporidia (red circle).
Capture Behavior and Stress Physiology
In the past decade, recreational shark fishing has become an increasingly popular activity because of the fighting ability of these strong animals and the “awe” and spectacle of catching a shark (Mcclellan Press et al. 2015). Most of these sharks are released alive (due to regulatory measures or conservation motives), with only a few species kept for consumption (e.g., Shortfin Mako and Common Thresher Sharks). However, there is a growing body of literature emphasizing that stressors associated with capture-and-handling elicit sublethal or lethal effects on sharks’ post-release (Skomal and Mandelman, 2012; Ellis et al. 2017; Whitney et al. 2017). Therefore, it is important to delineate which components of the capture event are most responsible for adversely impacting sharks. As a result, these can be mitigated via management frameworks and/or through education and outreach initiatives with the recreational fishing community. The Fisheries Research Foundation and M/V Albula provided the platform to collect a large amount of data pertaining to the effects of recreation fisheries capture on sharks. Specifically, we examined typical culprits observed in fishing practices (i.e., fight duration and handling time), individual traits (i.e., size and gender), and environmental conditions (i.e., water and air temperature), as well as capture behavior, which has received little attention despite its clear connection with energy expenditure/physiological consequences. More specifically, we examined the proportion of time individuals displayed burst-swimming behavior because of its large energetic cost that is also associated with anaerobic respiration and lactic acid production, which can lead to harmful levels of blood acidosis.
To measure capture behavior, we utilized tri-axial acceleration data collected by line-bourne accelerometers (Gulf Coast Data Concepts, LLC; Waveland, MS, USA) that recorded at 100Hz. These data have been preliminarily processed (Table 4; Fig. 7); ultimately, we plan to use time- series segmentation and cluster analyses to quantify periods of burst-swimming. This information when coupled with the rest of the data collected from each capture event (i.e., fishing practices and conditions, and individual traits), will then be compared to the blood chemistry of these animals both immediately after capture and post-handling, to identify which factor(s) had the most adverse impact on shark health. We are primarily concerned with changes in blood pH, metabolite levels, and ion concentrations, all of which can be indicative of capture stress and have sublethal or lethal outcomes if unresolved. These biomarkers were either measured in the field (e.g., i-STAT portable clinical analyzer and HemoCue Hb 201+), or on benchtop analyzers at the New England or New York Aquariums (e.g., NOVA Critical Care Xpress).
We collected 116.4 minutes (i.e., 700,000 observations that include 2,100,000 and 7,000 acceleration and depth/temperature data points, respectively) of capture behavior data from 24 individual sharks across six species (Fig. 7). In general, sharks exhibited an initial “fight or flight” escape response of burst-swimming towards depth, followed by an attenuation of activity and decrease in depth with time-on-hook. However, in the final moments of the fight and prior to leadering, most sharks displayed additional periods of burst-swimming behavior. Recorded fight durations ranged from 1 to 20 minutes across species, with common thresher and dusky shark exhibiting the longest and shortest average fight durations, respectively (Table 4). This may have been a product of species-specific behavior and/or a function of animal size, with captured common thresher sharks being much greater in size compared to dusky sharks.
The Common Thresher Shark exhibited the most intense capture behavior and was characterized by multiple “deep-dives” throughout the capture event and burst-swimming associated with the highest acceleration signal and vertical movement speeds of up to 4.7 m/s (Table 4). In addition, this species and Blacktip (though not captured in any of this species profiles) were the only two to display jumping behavior while hooked (associated with maximal energetic costs). Like Common Thresher Sharks, Sandbar Sharks exhibited several deep dives into cooler waters (20- 25 m), while Blacktip, Dusky, and Blue Sharks fought exclusively within the upper 10 m of the water column (Fig. 7). Though only one Blue Shark was captured, it was the only individual across species to not exhibit an initial escape response and remain at the immediate surface for most of the capture event (Fig. 7). And while the individual had the lowest peak acceleration signal (8.23 g’s), Common Thresher and Dusky Sharks had the highest recorded signals at 24.8 and 23.4 g’s, respectively. It is interesting that this individual could generate this high level of acceleration given their small size; however, when considering this species also exhibited the shortest fight durations, it points to this single period of burst-swimming leading to complete exhaustion of the animal.
Moving forward, the acceleration and depth/temperature time-series will be further analyzed to identify the proportion of time spent burst-swimming. This will then be coupled with blood chemistry values, which will be expanded upon in the coming weeks via analyses of plasma samples being stored at the New England Aquarium.
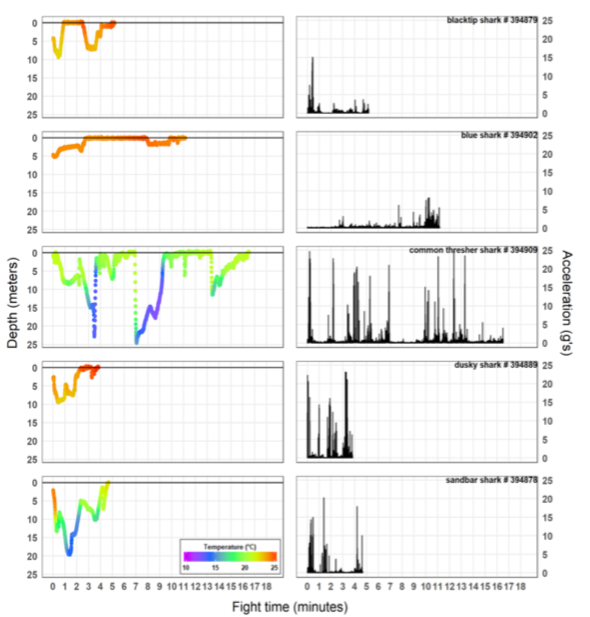
Figure 7. Time-series of depth (m; left panels) and vectoral sum acceleration (g’s; right panels) from five sharks representing the different species captured throughout the cruise. Water temperature (°C) is shown with depth observations using the color scale provided. Horizontal lines on depth time-series panels represent the water surface and are used to identify the approximate time each shark was leadered (i.e., end of capture event).
Conservation Applications and Proposed Future Work
The Fisheries Research Foundation and M/V Albula facilitated the most productive field season
for our science team so far regarding our objectives and brought together highly collaborative
research between five independent research institutions. Over the coming year the results from
this work will be communicated through multiple channels: scientific publications, popular press interviews, and aquarium outreach events. This will ensure data collected aboard the M/V Albula can be disseminated to a broad audience, ranging from scientific specialists to the general public.In this section, we propose future work that could be achieved with continued support of the Fisheries Research Foundation in 2020.
Shortfin Mako Sharks exhibit a high degree of variability in movements of individuals (Vaudo et al. 2017). Although a short-term track of an individual animal is of limited statistical value, it suggests the significance of the NY waters as a late summer foraging ground for this shark. While some previously tagged juveniles remained within the US EEZ and moved south during
the colder months, others have headed directly east into international waters (Camhi, LaBelle & Walters, unpublished data). This path of travel potentially exposes these young animals to mortality from commercial fishing efforts of several nations (Byrne et al. 2017; Queiroz et al. 2019). Therefore, the timing and direction of movement of this individual out of NY as ambient water temperatures drop will be of significant interest, and this track will be incorporated into a larger dataset for analysis. Understanding the drivers behind individual variability in movements for this species is essential due to recent stock assessments showing the North Atlantic population of Shortfin Mako Sharks to be overfished with fishing mortality well above sustainable levels (Rigby et al. 2019). We are seeking to purchase and deploy an additional 20 – 30 satellite tags over the next five years to gain further insight into large-scale, multi-year movements of this species.
To establish more robust estimates of migration and fine-scale habitat use, we currently have funds to support the deployment of an additional 90 acoustic transmitters in the following species: Sandbar Sharks (n = 20), Dusky Sharks (n = 30), Common Thresher Sharks (n = 20), Sand Tiger Sharks (n = 20). In addition, we have funds to support at least five additional years of the SBU extensive acoustic array network for collection of data from acoustically tagged sharks. Deployment of additional tags will provide a more rigorous assessment of critical habitat for these species in the MAB. Additional tagging will also allow for more robust conclusions regarding the timing and geographic extent of seasonal migrations, which remain elusive. Finally, combining acoustic telemetry data with capture behavior and physiology data will allow for better estimates of post release mortality that occurs from recreational fisheries capture. This wealth of information will directly inform state and federal level management of these vulnerable, but commonly targeted species.
We will continue to sample the tissues of migratory sharks to increase current sample sizes for target species; this will continue to improve inferences regarding foraging dynamics, ecotoxicology, and general health assessments. We will particularly focus our sampling efforts on Dusky Sharks, Common Thresher Sharks, Sand Tiger Sharks, and Blacktip Sharks in the MAB, as these species represent the most under sampled demographic of these projects to date. Through greater sampling effort in the coming years we plan to run additional assays, such as fatty acids, DNA, and additional contaminants (e.g., Polychlorinated Biphenyls [PCBs]) to provide a more holistic understanding of the foraging dynamics of MAB sharks, the implications for contaminant loading and shark health, and the associated impacts on human health.
The biggest challenge when examining capture behavior and stress physiology in sharks, is the inherent intraspecific differences that make having a large sample size necessary to characterize the response of species. For example, the profile from one Sandbar Shark (Fig. 8) indicates that this species exhibits a relatively weak capture behavior. However, another individual of nearly the same size and caught under the same conditions, displayed an entirely different and more exaggerated capture behavior (Fig. 8). To this end, larger sample sizes will account for these intraspecific differences and allow us to have a more robust understanding of how each species responds to both capture and handling. To date, Sandbar Sharks represent our largest sample size (n = 11 with acceleration and blood chemistry data) and is enough to begin looking at trends; however, some of the other species with two or less sharks, require additional animals before we can make any definitive conclusions.
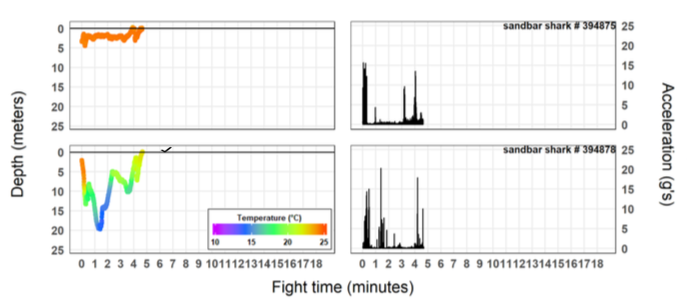
Figure 8. Time-series of depth (m; left panels) and vectoral sum acceleration (g’s; right panels) from two similar sized sandbar sharks (139 and 133 cm, respectively) captured with identical fishing practices and under similar environmental conditions. Water temperature (°C) is shown with depth observations using the color scale provided. Horizontal lines on depth time-series panels represent the water surface and we used to identify the approximate time each shark was leadered (i.e., end of capture event).
In addition, we would like to expand our capture behavior dataset by supplementing acceleration data with videography (via GoFish cameras). This past year we decided to hold off on using GoFish cameras because of water clarity and hopes of increasing our catch rates considering the sharks’ uncharacteristic reluctance towards taking the bait. Moving forward, we believe altering aspects of our terminal tackle (e.g., wind-on leaders, addition of tear-drop sinkers, and smaller- sized hooks) will permit the use of GoFish cameras without altering the fishability of our bait. In addition, recording specifications (e.g., “auto white balance” feature) can be manipulated to enhance viewability of our GoFish footage. Ultimately by incorporating this videography component in future work, it will permit a more fine-scale evaluation of capture behavior (e.g., tail beats, head shakes versus burst-swimming, and instances of regurgitation) and validation ofacceleration signals, while also providing valuable and exciting outreach material for collaborating parties.
Another consideration for this component of the project moving forward is the addition of fin- mounted acceleration data loggers (ADLs). ADL tag packages record high-resolution, tri-axial acceleration data for a user-defined period (up to three days) using a galvanic timed release (Fig. 9). These tags then float to the surface and can be recovered using a Yagi antenna to locate the ping from uniquely coded VHF transmitters embedded in each ADL tag package. Because of the known orientation of the fin-mounted accelerometer relative to shark’s body, these data can then be translated into movement metrics such as the orientation of the shark and individual tail beats, all of which can be used to evaluate recovery periods and instances of post-release mortality (e.g., Whitney et al. 2017; Knotek et al. unpublished data). This would provide us with an unparalleled, start-to-finish evaluation of how recreational fishing impacts sharks in the northeast U.S. The Fisheries Research Foundation’s M/V Albula provides the ideal platform for this type of work because of its ability to launch and operate multiple smaller-sized vessels simultaneously.
Literature Cited and Further Reading
- Adams, D. H., & McMichael Jr, R. H. (1999). Mercury levels in four species of sharks from the Atlantic coast of Florida. Fishery Bulletin, 97(2), 372-379.
- Byrne, M. E., Cortés, E., Vaudo, J. J., Harvey, G. M., Sampson, M., Wetherbee, B.M., et al. (2017). Satellite telemetry reveals higher fishing mortality rates than previously estimated, suggesting overfishing of an apex marine predator. Proceedings of the Royal Society B, 284, 20170658.
- Cailliet, G. M., Cavanagh, R. D., Kulka, D. W., Stevens, J. D., Soldo, A., Clo, S., et al. (2009). Isurus oxyrinchus. The IUCN Red List of Threatened Species 2009: e.T39341A10207466.
- http://dx.doi.org/10.2305/IUCN.UK.2009-2.RLTS.T39341A10207466.en. Downloaded on 30 November 2017
- Camhi, M. D., Pikitch, E. K, & Babcock, E. A. (2008). Sharks of the Open Ocean: Biology, Fisheries, and Conservation. Blackwell Publishing, Oxford, UK.
- Camhi, M. D., Valenti, S. V, Fordham, S. V., Fowler S. J., & Gibson, C. (2009). The Conservation Status of Pelagic Sharks and Rays: Report of the IUCN Shark Specialist Pelagic Shark Red List Workshop. IUCN Species Survival Commission Shark Specialist Group. Newbury, UK.
- Casey, J. G., & Kohler, N. E. (1992). Tagging studies on the Shortfin Mako Shark (Isurus oxyrhynchus) in the western North Atlantic. Australian Journal of Marine and Freshwater Research, 43, 45-60.
- Crump, K. L., & Trudeau, V. L. (2009). Mercury‐induced reproductive impairment in fish. Environmental Toxicology and Chemistry, 28(5), 895-907.
- Curtis, T. H., et al. 2019. First insights into the movements of young-of-the-year white sharks (Carcharodon carcharias) in the western North Atlantic Ocean. Scientific Reports, 8, 10794.
- Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, P. M., Harrison, L. R., et al. (2014). Extinction risk and conservation of the world’s sharks and rays. Elife, 3, e00590.
- Ellis, J. R., McCully Phillips, S. R., & Poisson, F. (2017). A review of capture and post‐release mortality of elasmobranchs. Journal of Fish Biology, 90(3), 653-722.
- Engle, M. A., Tate, M. T., Krabbenhoft, D. P., Schauer, J. J., Kolker, A., Shanley, J. B., et al. (2010). Comparison of atmospheric mercury speciation and deposition at nine sites across central and eastern North America. Journal of Geophysical Research: Atmospheres, 115(D18).
- Ferretti, F., Worm, B., Britten, G. L., Heithaus, M. R., & Lotze, H. K. (2010). Patterns and ecosystem consequences of shark declines in the ocean. Ecology letters, 13(8), 1055-1071.
- Frisk, M. G., Shipley, O. N., Martinez, C. M., McKown, K. A., Zacharias, J. P., & Dunton, K. J. (2019). First observations of long‐distance migration in a large skate species, the winter skate: Implications for population connectivity, ecosystem dynamics, and management. Marine and Coastal Fisheries, 11(2), 202-212.
- Goldman, K. J., Baum, J., Cailliet, G. M., Cortés, E., Kohin, S., Macías, D., et al. (2009). Alopias vulpinus. The IUCN Red List of Threatened Species 2009: e.T39339A10205317.
- http://dx.doi.org/10.2305/IUCN.UK.2009-2.RLTS.T39339A10205317.en. Downloaded on 30 November 2017.
- Graham, F., Rynne, P., Estevanez, M., Luo, J., Ault, J. S., & Hammerschlag, N. (2016). Use of marine protected areas and exclusive economic zones in the subtropical western North Atlantic Ocean by large highly mobile sharks. Diversity and Distributions, 22(5), 534-546.
- Hammerschlag, N., et al. (2019). Disappearance of white sharks leads to the novel emergence of an allopatric apex predator, the sevengill shark. Scientific Reports, 9, 1908.
- Heithaus, M. R., Frid, A., Wirsing, A. J., & Worm, B. (2008). Predicting ecological consequences of marine top predator declines. Trends in Ecology & Evolution, 23(4), 202-210.
- Howey, L. A., Wetherbee, B. M., Tolentino, E. R., Howey, L. A., & Shivji, M. S. (2017). Biogeophysical and physiological processes drive movement patterns in a marine predator. Movement ecology, 5(1), 16.
- Hussey, N. E., MacNeil, M. A., Olin, J. A., McMeans, B. C., Kinney, M. J., Chapman, D. D., et al. (2012). Stable isotopes and elasmobranchs: tissue types, methods, applications and assumptions. Journal of Fish Biology, 80(5), 1449-1484.
- Kim, S. L., del Rio, C. M., Casper, D., & Koch, P. L. (2012). Isotopic incorporation rates for shark tissues from a long-term captive feeding study. Journal of Experimental Biology, 215(14), 2495-2500.
- Kiszka, J. J., Aubail, A., Hussey, N. E., Heithaus, M. R., Caurant, F., & Bustamante, P. (2015). Plasticity of trophic interactions among sharks from the oceanic south-western Indian Ocean revealed by stable isotope and mercury analyses. Deep Sea Research Part I: Oceanographic Research Papers, 96, 49-58.
- Kwon, S. Y., Blum, J. D., Chen, C. Y., Meattey, D. E., & Mason, R. P. (2014). Mercury isotope study of sources and exposure pathways of methylmercury in estuarine food webs in the Northeastern US. Environmental Science and Technology, 48(17), 10089-10097.
- Le Bourg, B., Kiszka, J., & Bustamante, P. (2014). Mother–embryo isotope (δ15N, δ13C) fractionation and mercury (Hg) transfer in aplacental deep‐sea sharks. Journal of fish biology, 84(5), 1574-1581.
- Lyons, K., Kacev, D., Preti, A., Gillett, D., Dewar, H., & Kohin, S. (2019). Species-specific characteristics influence contaminant accumulation trajectories and signatures across ontogeny in three pelagic shark species. Environmental science & technology.
- Mandelman, J. W., Cooper, P. W., Werner, T. B., & Lagueux, K. M. (2008). Shark bycatch and depredation in the US Atlantic pelagic longline fishery. Reviews in Fish Biology and Fisheries, 18(4), 427.
- Matulik, A. G., Kerstetter, D. W., Hammerschlag, N., Divoll, T., Hammerschmidt, C. R., & Evers, D. C. (2017). Bioaccumulation and biomagnification of mercury and methylmercury in four sympatric coastal sharks in a protected subtropical lagoon. Marine pollution bulletin, 116(1- 2), 357-364.
- Maz-Courrau, A., López-Vera, C., Galvan-Magaña, F., Escobar-Sánchez, O., Rosíles-Martínez, R., & Sanjuán-Muñoz, A. (2012). Bioaccumulation and biomagnification of total mercury in four exploited shark species in the Baja California Peninsula, Mexico. Bulletin of Environmental Contamination and Toxicology, 88(2), 129-134.
- McCauley, D. J., Young, H. S., Dunbar, R. B., Estes, J. A., Semmens, B. X., & Micheli, F. (2012). Assessing the effects of large mobile predators on ecosystem connectivity. Ecological Applications, 22(6), 1711-1717.
- Mcclellan Press, K., Mandelman, J., Burgess, E., Cooke, S. J., Nguyen, V. M., & Danylchuk, A. J. (2016). Catching sharks: recreational saltwater angler behaviours and attitudes regarding shark encounters and conservation. Aquatic Conservation: Marine and Freshwater Ecosystems, 26(4), 689-702.
- Melnychuck, M. C., et al. (2017). Informing conservation strategies for the endangered Atlantic sturgeon using acoustic telemetry and multi‐state mark–recapture models. Journal of Applied Ecology, 54, 3, 914-925.
- Merson, R. R., & Pratt, H. L. (2001). Distribution, movements and growth of young sandbar sharks, Carcharhinus plumbeus, in the nursery grounds of Delaware Bay. Environmental Biology of Fishes, 61(1), 13-24.
- Mull, C. G., Blasius, M. E., O’Sullivan, J. B., & Lowe, C. G. (2012). Heavy metals, trace elements, and organochlorine contaminants in muscle and liver tissue of juvenile White Sharks, Carcharodon carcharias, from the Southern California Bight. Global perspectives on the biology and life history of the white shark, 59-75.
- Musick, J.A., Grubbs, R.D., Baum, J. & Cortés, E. (2009). Carcharhinus obscurus. The IUCN Red List of Threatened Species 2009: e.T3852A10127245. http://dx.doi.org/10.2305/IUCN.UK.2009-2.RLTS.T3852A10127245.en. Downloaded on 30 November 2017.
- O’Bryhim, J. R., Adams, D. H., Spaet, J. L., Mills, G., & Lance, S. L. (2017). Relationships of mercury concentrations across tissue types, muscle regions and fins for two shark species. Environmental Pollution, 223, 323-333.
- Queiroz, N., Humphries, N. E., Couto, A., Vedor, M., da Costa, I., Sequeira, A. M., et al. (2019). Global spatial risk assessment of sharks under the footprint of fisheries. Nature, 572(7770), 461- 466.
- Rechisky, E. L., & Wetherbee, B. M. (2003). Short-term movements of juvenile and neonate sandbar sharks, Carcharhinus plumbeus, on their nursery grounds in Delaware Bay. Environmental Biology of Fishes, 68(2), 113-128.
- Rigby, C.L., Barreto, R., Carlson, J., Fernando, D., Fordham, S., Francis, M.P., et al. (2019). Isurus oxyrinchus. The IUCN Red List of Threatened Species 2019: e.T39341A2903170. http://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS.T39341A2903170.en. Downloaded on 17 September 2019.
- Roff, G., Brown, C. J., Priest, M. A., & Mumby, P. J. (2018). Decline of coastal apex shark populations over the past half century. Communications Biology, 1(1), 223.
- Shipley, O. N., Brooks, E. J., Madigan, D. J., Sweeting, C. J., & Grubbs, R. D. (2017). Stable isotope analysis in deep-sea chondrichthyans: recent challenges, ecological insights, and future directions. Reviews in Fish Biology and Fisheries, 27(3), 481-497.
- Shipley, O. N., Gallagher, A. J., Shiffman, D. S., Kaufman, L., & Hammerschlag, N. (2019). Diverse resource-use strategies in a large-bodied marine predator guild: evidence from differential use of resource subsidies and intraspecific isotopic variation. Marine Ecology Progress Series, 623, 71-83.
- Skomal, G. B., & Mandelman, J. W. (2012). The physiological response to anthropogenic stressors in marine elasmobranch fishes: a review with a focus on the secondary response. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 162(2), 146-155.
- Stevens, J. 2009. Prionace glauca. The IUCN Red List of Threatened Species 2009: e.T39381A10222811. http://dx.doi.org/10.2305/IUCN.UK.2009-2.RLTS.T39381A10222811.en. Downloaded on 30 November 2017.
- Hammerschlag, N., Schmitz, O. J., Flecker, A. S., Lafferty, K. D., Sih, A., Atwood, T. B., et al. (2019). Ecosystem function and services of aquatic predators in the Anthropocene. Trends in Ecology & Evolution, 34(4), 369-383.
- Vaudo, J. J., Wetherbee, B. M., Wood, A. D., Weng, K., Howey-Jordan, L. A., Harvey, G. M., & Shivji, M. S. (2016). Vertical movements of shortfin mako sharks Isurus oxyrhynchus in the western North Atlantic are strongly influenced by temperature. Marine Ecology Progress Series, 547,163-175.
- Vaudo, J. J., Byrne, M. E., Wetherbee, B. M., Harvey, G. M., & Shivji, M. S. (2017). Long‐term satellite tracking reveals region‐specific movements of a large pelagic predator, the shortfin mako shark, in the western North Atlantic Ocean. Journal of Applied Ecology, 54, 1765-1775.
- Whitney, N. M., White, C. F., Anderson, P. A., Hueter, R. E., & Skomal, G. B. (2017). The physiological stress response, postrelease behavior, and mortality of blacktip sharks
- (Carcharhinus limbatus) caught on circle and J-hooks in the Florida recreational fishery. Fishery Bulletin, 115(4), 532-544.
- Worm, B., Davis, B., Kettemer, L., Ward-Paige, C. A., Chapman, D., Heithaus, M. R., et al. (2013). Global catches, exploitation rates, and rebuilding options for sharks. Marine Policy, 40, 194-204.
- Zeichner, S. S., Colman, A. S., Koch, P. L., Polo-Silva, C., Galván-Magaña, F., & Kim, S. L. (2017). Discrimination factors and incorporation rates for organic matrix in shark teeth based on a captive feeding study. Physiological and Biochemical Zoology, 90(2), 257-272.
